TL;DR immunity is a difficult concept, requires tons of reading and may need an actual human mediator to explain this. The field of immunology enables us to understand why some infections can be fatal, but some don’t. Here we explore some of the explanations for both.
Recently I was asked this question:
Why do we still get flu/fever even though we had it before? Don’t our bodies develop immunity against diseases if we are infected (vaccine concept)?
At first, I thought I could simply answer this question in 1 or 2 sentences, but then a realization struck me rather deeply. An epiphany that muttered “this is going to be a lecture”. It is now making its way here on Caspershire without me realizing it.
I figured that if this warranted a blog post, it better be (1) structured, with (2) examples, and (3) almost-complete explanation. So, let’s get started.
Concept of Immunity
First of all before I delve deeper, this field is huge and complicated. Collectively, scientists have accumulated massive man-hours in order to understand it, but the general sentiment is that our knowledge is still far from being 100%. If we know even 50-60% of this whole thing, we might have been able develop good vaccines against most diseases.
To give you a taste of it, even the latest vaccine trial for HIV (RV144 study) came with more questions than answers. Other than HIV, we are still struggling to create universal influenza vaccine, and we still depend on annual vaccination, which also does not have the best efficacy (but it works, and you should still get it).
Here is a brief recap on how immunity, and some extent the vaccination, works. First, an insult (e.g. virus) enters our system. Our body has sensors to detect it, and once they do, they raise alarms. There are many kinds of alarms, such as:
- Raising body temperature to kill the virus (a.k.a fever).
- A subset of specialized immune cells eat that virus, chew it into pieces, and present the pieces to another subset of specialized immune cells.
- Initiating local inflammation to encourage more immune cells to combat the invading virus.
- Days after the initial infection, our body starts producing antibodies to target and degrade the virus.
These 4 example countermeasures may look simple, but I can tell you that there are tons of players involved in this process, on the scale of hundreds to thousands. The anime Cells at Work (Hataraku Saibo) can give you a better illustration at this.
The step number 4 is, perhaps, the most important part in establishing protection against future infection by the same virus. Antibody, which is produced by mature B cells (a.k.a plasma cells), is a small protein that can target viruses and tag them for destruction. The presence of antibody makes a person immune to infection, or if that person still got infected, s/he won’t be sick for too long.
In other words, for protection to develop, our body needs to see it first. This is the scientific basis for immunity and vaccination. For the sake of brevity, we won’t be discussing vaccination here.
Exhibit A: The Spanish Flu 1918
The Spanish Flu 1918 (Influenza A virus (IAV), H1N1 subtype) was a significant pandemic in the history of infectious diseases. It is dubbed as the Mother of All Pandemics (reviewed by JK Taubenberger & DM Morens, 2006). It was estimated 1/3 of the world’s population (roughly 500 million persons) were afflicted during the pandemic with fatality rate hovering around 10%, which was really high for influenza.
This was a significant event that intertwined with the chaos that the first world war brought upon us. The outbreak did not start in Spain, but because the Spaniards were vocal about sharing information regarding the outbreak, it was known as the Spanish Flu. Other nations did not share much, fear such panic would affect the morale of the soldiers.
So, what did go wrong with the Spanish Flu? Let’s look at this chart, adapted from Taubenberger’s paper:
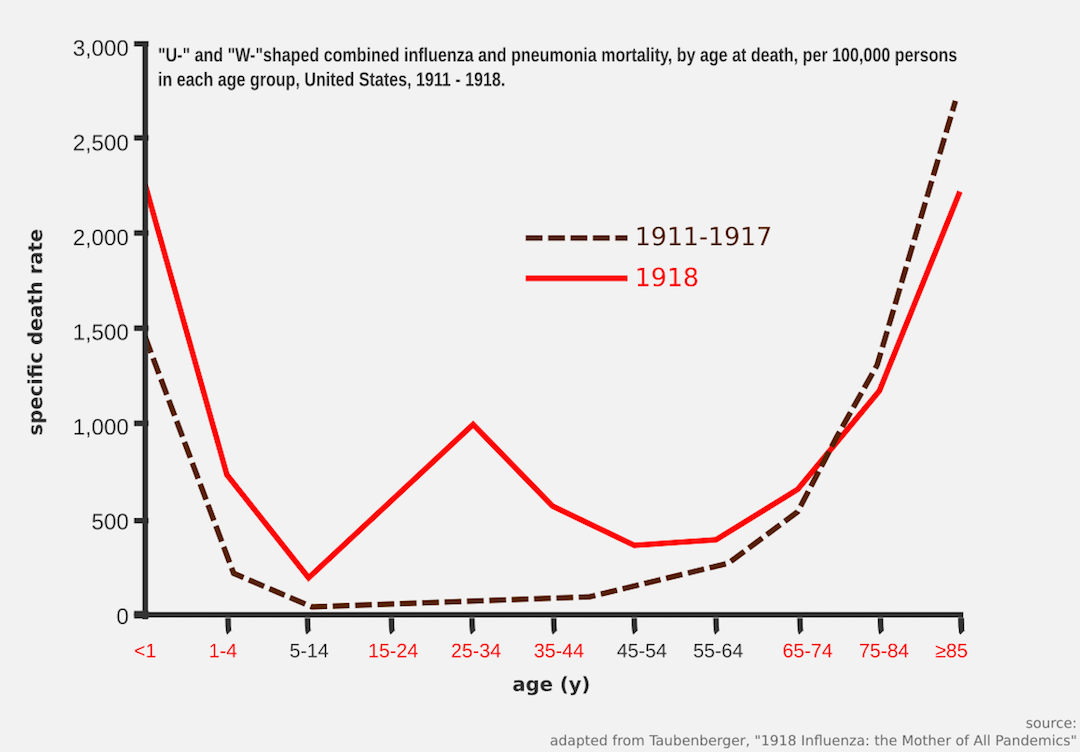
Typically, whenever influenza A outbreak happens, usually two age groups that are going to be hit the hardest: babies and elderlies. Although babies are capable to mount defense against infection, they usually have hard time to cope with the changes in physiological responses.
In other words, babies can initiate the process to make antibodies against the infection (indicating potent immunological response in neonates), but the heat from the inflammation and fever would be a little too harsh for them (signifying a weaker physiological response). Note that I am using the words immunological and physiological response loosely here. As for the elderlies, the immune system does age along with the host, a condition termed as immune senescence.
Now, let’s get back to the chart. The obvious question here is why young adults (20 – 40 y/o) were also affected? Does this not contradict the statement I just made about babies and elderlies? Aren’t young adults supposed to be healthier since they have a much more robust immune and physiological response?
Well, they are many potential answers to this, and each of the answer might contribute equally to the severity of the infection. However, there is one explanation that captivated me: immune imprinting. Before we dissect this, there is one more thing we need to understand about influenza A virus.
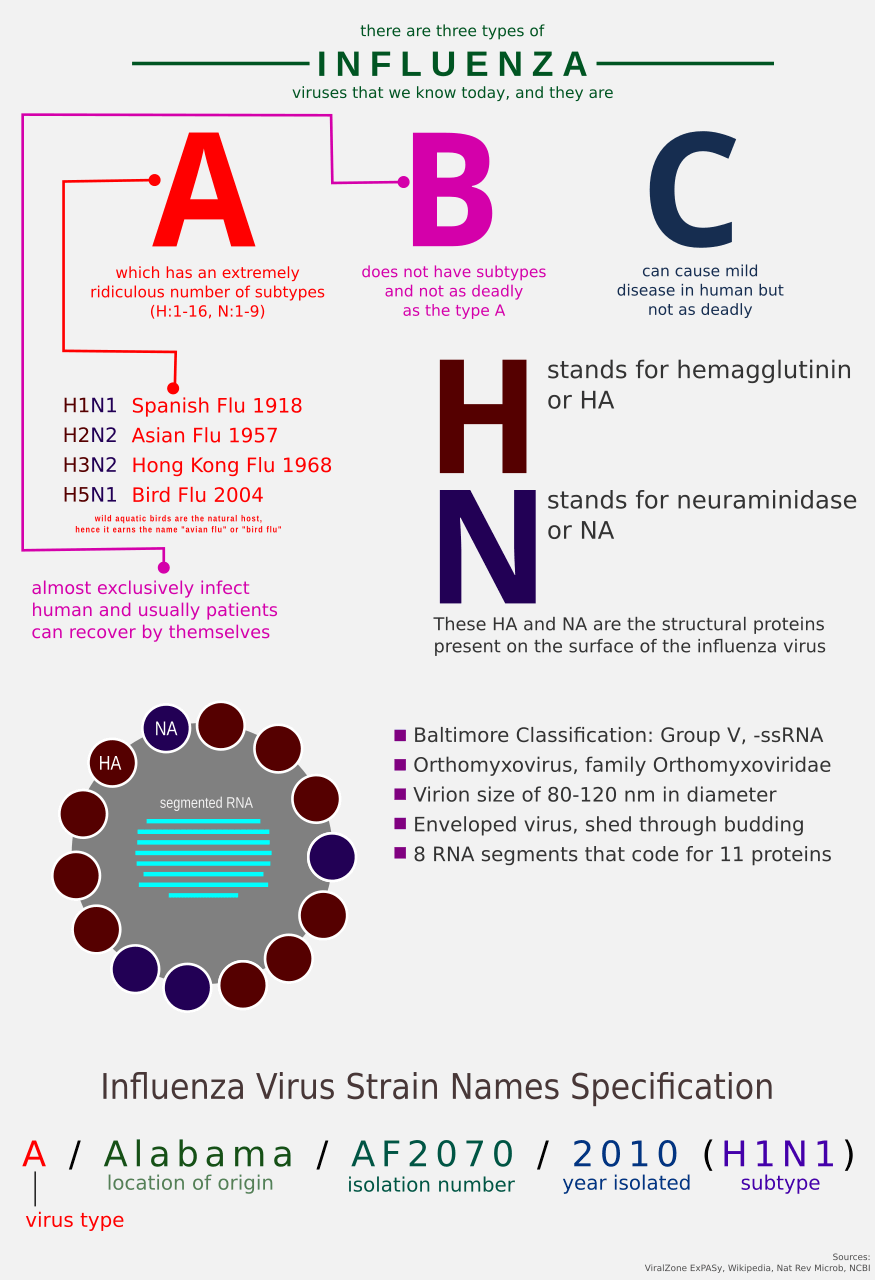
There are many kinds of them.
In news, reports about influenza A outbreak are often accompanied by viral identification such as “H1N1”, or “H3N2”, or “H5N1”. What are these? Like individual units of human species are identified by their outward appearances such as facial feature, things we wear, etc., viruses have their own identifiers based on their outward appearances too.
For influenza A virus, they have 2 proteins that decorate their external surface, the Hemagglutinin and the Neuraminidase. There are 11 known subtypes of H (H1, H2, …, H11) and 9 known subtypes of N (N1, N1, …, N9).
Okay, let’s go back to the issue at hand: imprinting. So, what about it? According to a blog post written by Dr. Francis Collins, birth year predicts bird flu risk. How so? Well, the premise is simple: the first influenza infection that you had, probably the one that your immune system remembers the most.
For example, if you were first exposed to the influenza A strain H1N1 when you were child, there is a good probability that after a few decades later, a related H1N1 outbreak will not cause harm to you. But if you were exposed to H3N2 when you were child, an outbreak caused by H1N1 would probably be something of a concern.
“Did this happen with the Spanish Flu 1918, this imprinting thing?”
People seem to think so, among other factors (Worobey et al., 2014).
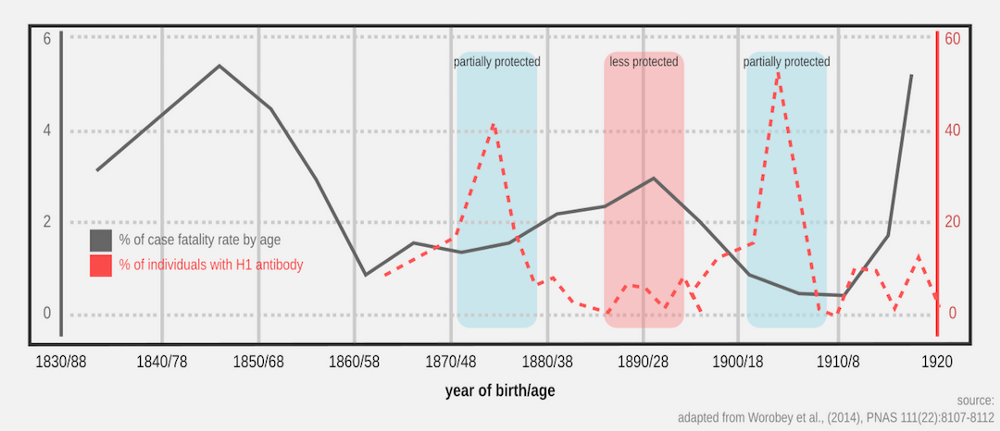
There is a trend in the chart above: higher amount of H1 antibody (red dotted lines) correlates with lower fatality rate by age (dark line). The trend seems cyclical, repeating every few decades or so. The age label on the X-axis means age in 1918.
For a person that was born early 1900, s/he would be first exposed to H1N1 strain that was circulating prior to the pandemic version of the same strain. In other words, prior exposure to H1 subtype just before the pandemic took place conferred partial protection against the stronger, pandemic version of the same strain.
(This is the definition of vaccination, where the immune system got to know its nemesis and can formulate a winning strategy for another re-match a few decades later.)
Being born a decade before (1890) when the dominant circulating influenza A was not H1, the amount of antibody against H1 was lower in the coming decades (if not nonexistent), thus higher mortality in ~28 y/o young adults when the pandemic hit. This was how 1918 dragged many of the young adults into grave. Of course, other factors could be playing as well.
Again, I must stress that the explanation I am giving here is simplified. I would like to write more because there are few more grounds I would like to cover (e.g. cytokine storm and original antigenic sin), but by doing so I am risking this note to become a novel.
The Many Different Facades
The example in the exhibit A above demonstrates how immunological memories could last long to a fault, there are aspects of our immune system that can’t even progress into producing protective antibodies for a long period. Viruses, for example, mutate so fast that even though a person has been exposed before to a certain specific strain of a certain specific virus, immune machineries probably can’t take on it head-on the next time it sees it.
The analogy goes like this: it would be challenging to recognize that one cousin during big family gathering because s/he keeps changing his/her hair color, trying new glass frame every 2 weeks, and wearing RGB braces with different color combination that’s changed on daily basis.
This is one of the reasons why it is hard to make vaccines against HIV (among other factors) because it mutates so rapidly that our immune system can’t keep up with it.
Our Immunity
Concerning viral infections, sometimes the troubles don’t come from the viruses themselves. Virus infections are generally and mostly self-limited, meaning that they are rarely fatal. It was hypothesized that even pandemic strains couldn’t and wouldn’t actually kill you (Brundage & Shanks, 2008). So, what would kill people during an influenza outbreak?
The bacteria that are already present in the body, and sometimes the body itself. In other words, you would kill yourself if IAV infected you. In the case of the immune system gone awry, this is where I am going to cover briefly on a phenomenon known as the cytokine storm.
First, what are the cytokines? These are small proteins that are generally involved in alerting the immune system that there is something very wrong going on. For example when the IAV hits the lung, cells in the lung are going to release cytokines, and these cytokines travel across the body to call for help. As the result, a battalion of immune soldiers marching to the lung to deter and contain the invasion.
However, unregulated production of cytokines is not ideal. Enter, cytokine storm, where the local inflammation that should be contained in the lung spills into other organ systems excessively and affecting the entire body (Tisoncik et al., 2012). Cytokine storm can be fatal. It can cause multiple organ failures (e.g. acute kidney failure to a point requiring hemodialysis) and low blood pressure due to internal bleeding (hemorrhage).
Now we are switching gear to the role of opportunistic bacteria during a viral infection.
Just so everyone is on the same page here, we have more bacterial cells in our body than our own cells (this fact is somehow controversial and the number may be affected by defecation event). Some of them help us, some of them just sitting there doing nothing.
Some of them are waiting to spring into action once they sensed the host has been weakened. These are what we call the opportunistic pathogens.
In case of influenza infection (viral), a person may further develop pneumonia, which is a bacterial infection in the lung. Since our body is weakened by the influenza, our body has less available resources to ward off other threats, such as S. pneumoniae, the popular causative agent for pneumonia. The evidence of bacterial infection and colonization in the lung usually manifests itself as coughing accompanied by thick green/yellow phlegm.
Even though our immune system would struggle to contain both threats at the same time, proper interventions can resolve both infections. However in extreme cases, the local bacterial infection in the lung could progress to systemic bacterial infection when they invade the bloodstream and spread to other organ systems, a condition termed as sepsis.
So, when you have fever and you cough up thick green phlegm, seek doctor as soon as possible.
Recap
This whole thing ain’t easy. What I just wrote here might be the first page of a 10,000-pages encyclopedia.